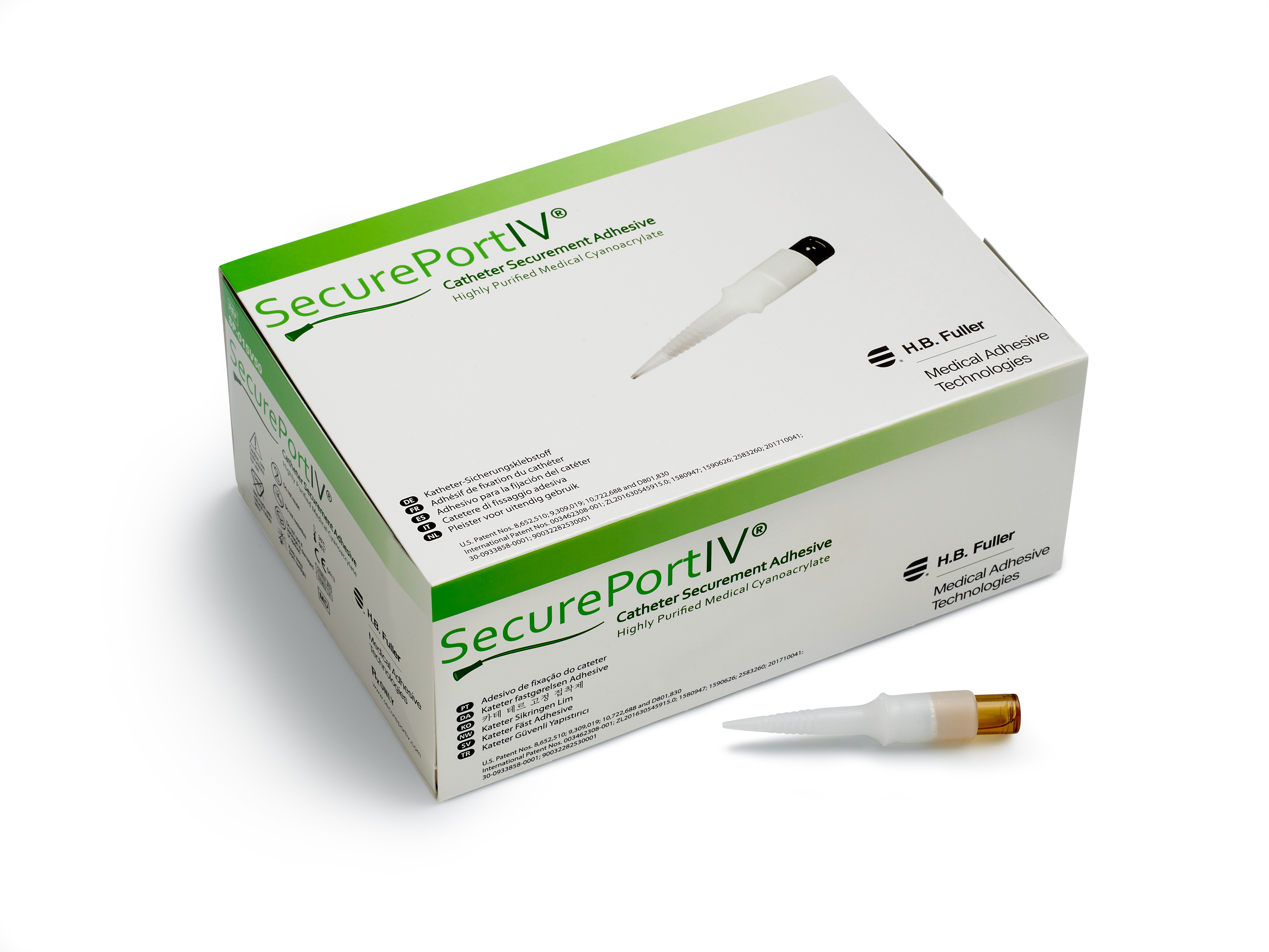
SecurePortIV®
Every line, every time
Catheter Securement Adhesive
The most versatile care and maintenance device in vascular access.
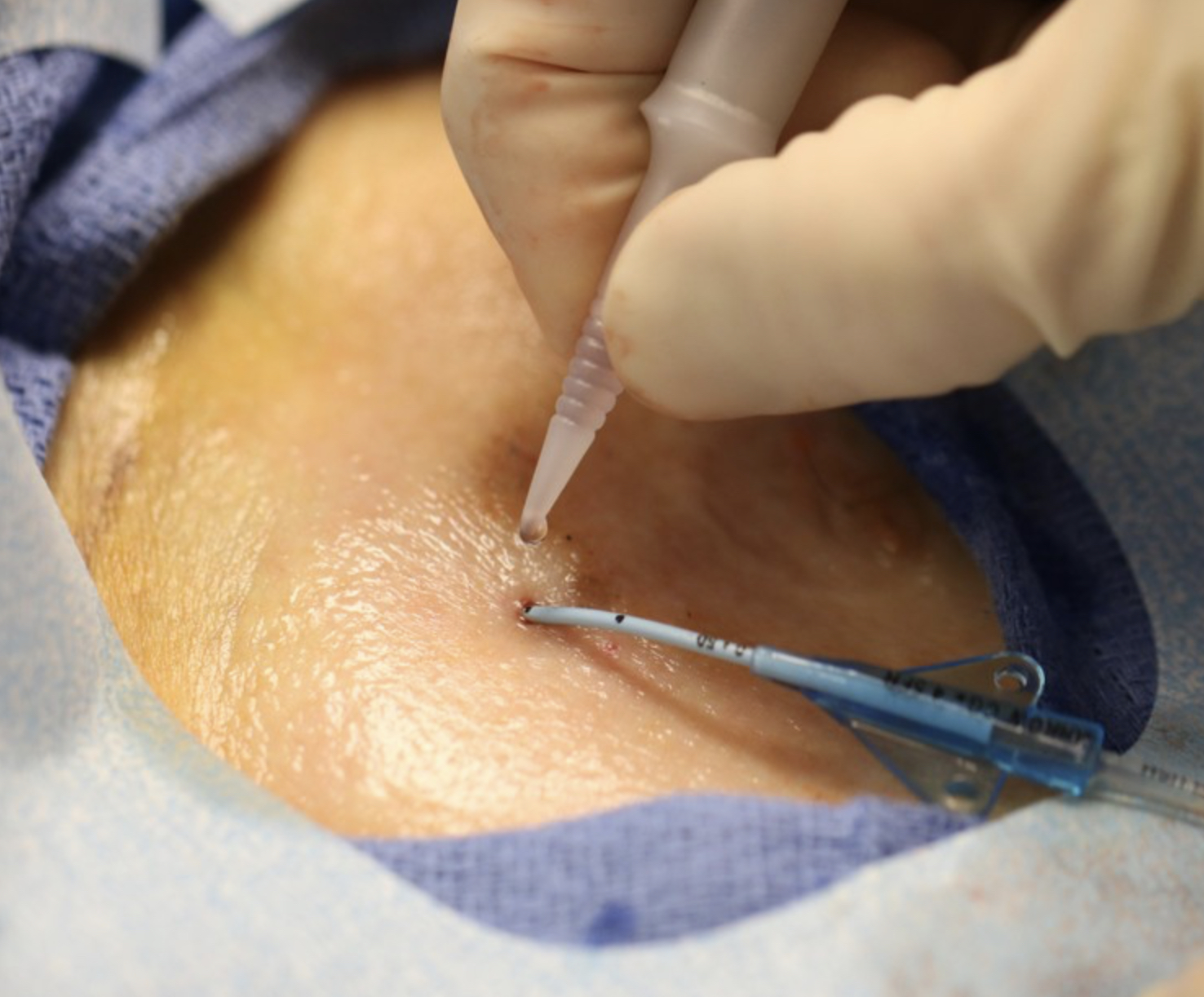
SecurePortIV® adhesive combines multiple catheter care and maintenance requirements into a single product. It is the only FDA-cleared liquid adhesive that secures the catheter to the skin at both the insertion site and the hub to reduce catheter movement, migration and dislodgement. SecurePortIV adhesive also seals the insertion site to protect it from microbial contamination and to reduce bleeding and oozing that is commonly associated with unscheduled dressing changes.1

Securement
Sealant
Infection Prevention
Versatility
Features & Benefits
- Securement
Secures the catheter to the skin at both the insertion site and the hub to reduce catheter movement, migration and dislodgement. - Sealant
Clinical studies suggest cyanoacrylate skin adhesives like SecurePortIV® completely seal the insertion site, helping to minimize non-routine dressing changes. - Infection Prevention
Formulation reported to exhibit activity against gram-negative and gram-positive bacteria, yeast, and fungi commonly associated with catheter related bloodstream infections, eliminating greater than 8-logs after 3 minutes of contact in in-vitro studies - Versatility
Suitable for all catheters and patients of any age. It’s time to protect every vascular access catheter. - Provides incremental securement to dressing3
- Immobilizes skin flora that has not been removed by standard skin prep
- Can be used on all lines and patients of all ages
- Allows site visualization after application
- Provides water barrier
- 100% sterile
- Product Code: SP-015V50 | 0.15ml / 50qty per box
Vascular access
Featured Products
Explore our full range of both internal and external medical-grade solutions and discover how we’re redefining performance in wound closure, vascular access, liquid embolics and infection control.
Our Associations


References
1 Kleidon, et al. A Pilot Randomized Controlled Trial of Novel Dressing and Securement Techniques in 101 Pediatric Patients. J Vasc Interv Radiol. 2017 Sep 18
2 Prince, et al. Immobilization and Death of Bacteria by Flora Seal® Microbial Sealant. International Journal of Pharmaceutical Science Invention, 2017.
3 Data on file. Average securement strength calculated from measured values. Nov/Dec 2016.