SurgiSeal®
Superior flexibility and optimal strength
Topical Skin Adhesive
Superior flexibility and optimal strength.
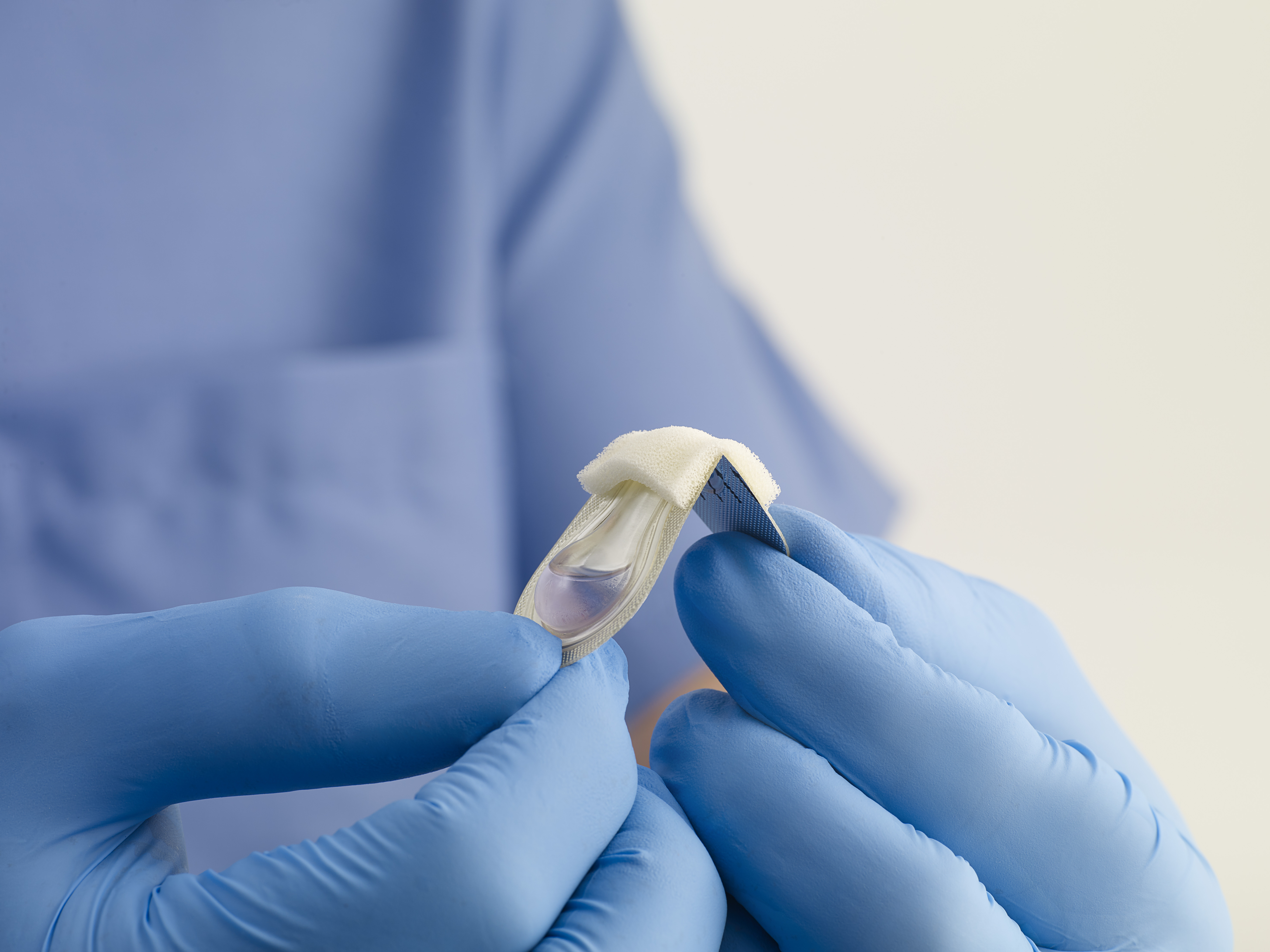
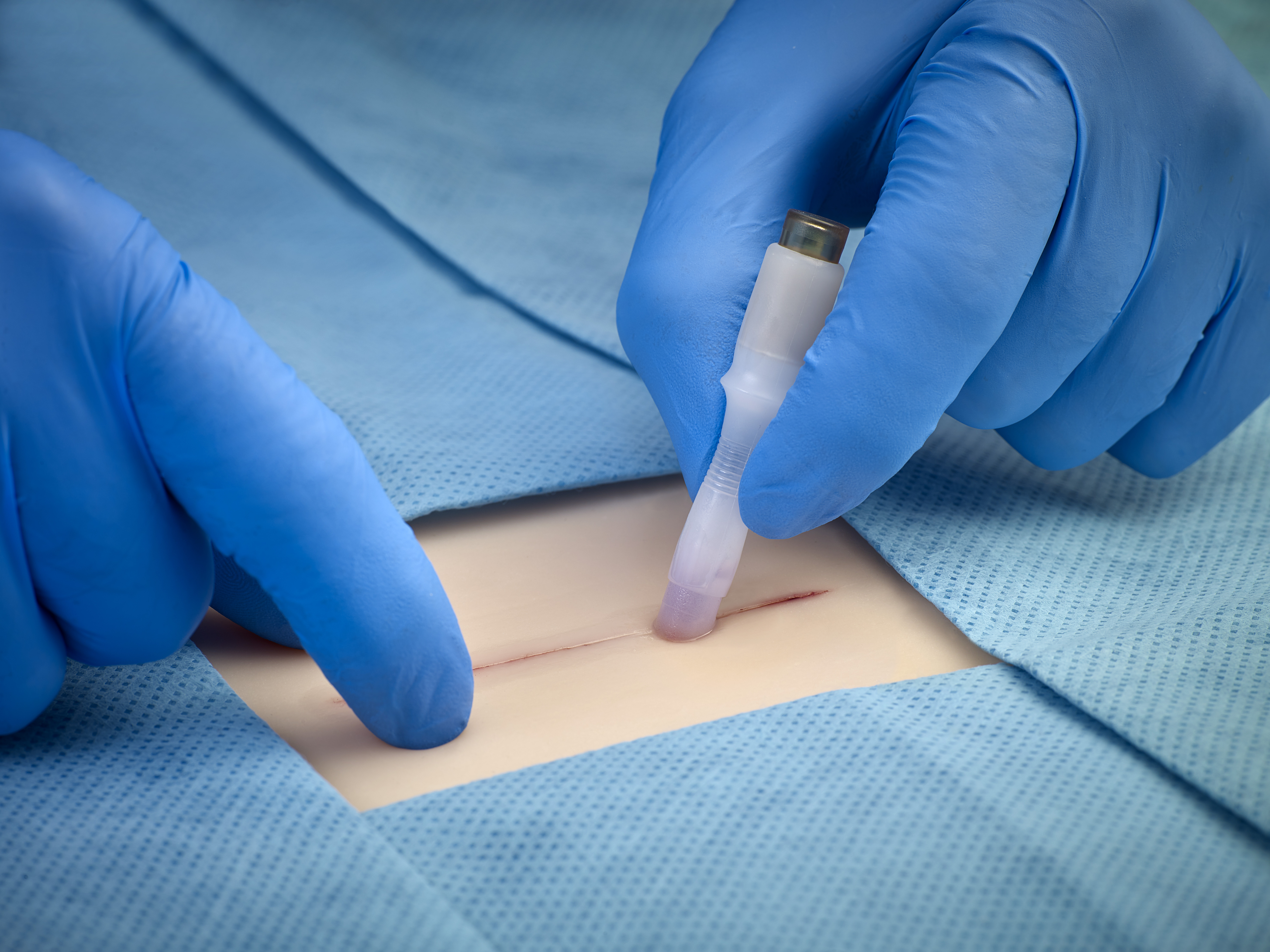
SurgiSeal® Topical Skin Adhesive is a high-performance topical skin adhesive designed to offer both clinical efficiency and patient comfort. It is the only topical skin adhesive in the world to receive FDA 510k clearance in demonstrating inhibition of gram-positive and gram-negative bacteria growth1. Our proprietary formula does not require an initiator to activate and provides a flexible, water-resistant, antimicrobial protective coating.
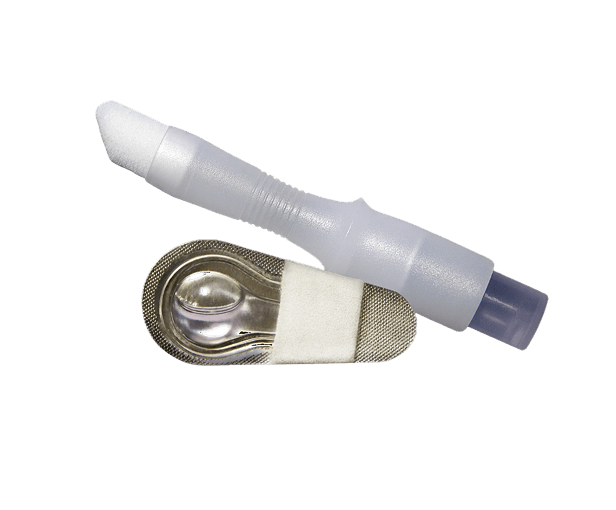
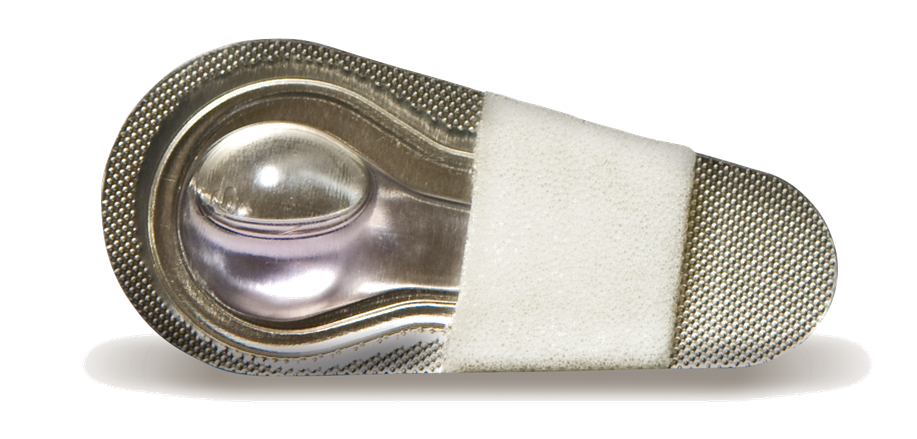
SurgiSeal® adhesive has two unique applicator options. The SurgiSeal Stylus® applicator has 0.5ml of adhesive and offers an ergonomic design. This patented applicator allows for maximum coverage with minimal adhesive material. The original SurgiSeal® applicator has 0.35ml of adhesive and a wider foam tip that delivers consistent and generous coverage of adhesive.

Inhibition of Bacteria
High-Strength Formulation
OctylFlex® Technology
Distinct Applicator Options
Features & Benefits
- Inhibition of Bacteria
SurgiSeal® is the only topical skin adhesive in the world to receive FDA 510K clearance in demonstrating inhibition of gram positive and gram negative bacteria growth.1 - High-Strength Formulation
Made with a 2-octyl cyanoacrylate formulation, which features a greater breaking strength and broader uses in clinical applications than existing n-butyl cyanoacrylates or octyl/butyl blends. 3 - OctylFlex® Technology
Our unique formulation provides the optimal balance of properties that matter most to healthcare providers while providing the best clinical outcome for patients: optimal flexibility, broad spectrum antimicrobial protection, an integrated catalyst, adhesive purity, and improved patient comfort. - Distinct Applicator Options
Available in multiple formats in both 0.35ml and 0.5ml applicators for tailored application needs. - In-vitro studies have shown that gram-negative and gram-positive bacteria do not proliferate within the SurgiSeal adhesive. Moreover, SurgiSeal adhesive demonstrates a log reduction of greater than seven in organisms including Methicillin-Resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Corynebacterium pseudodiphtheriticum, Klebsiella pneumoniae, Escherichia coli, and Pseudeomonas Aeruginosa.2
- High Moisture Vapor Transmission Rate (MVTR)
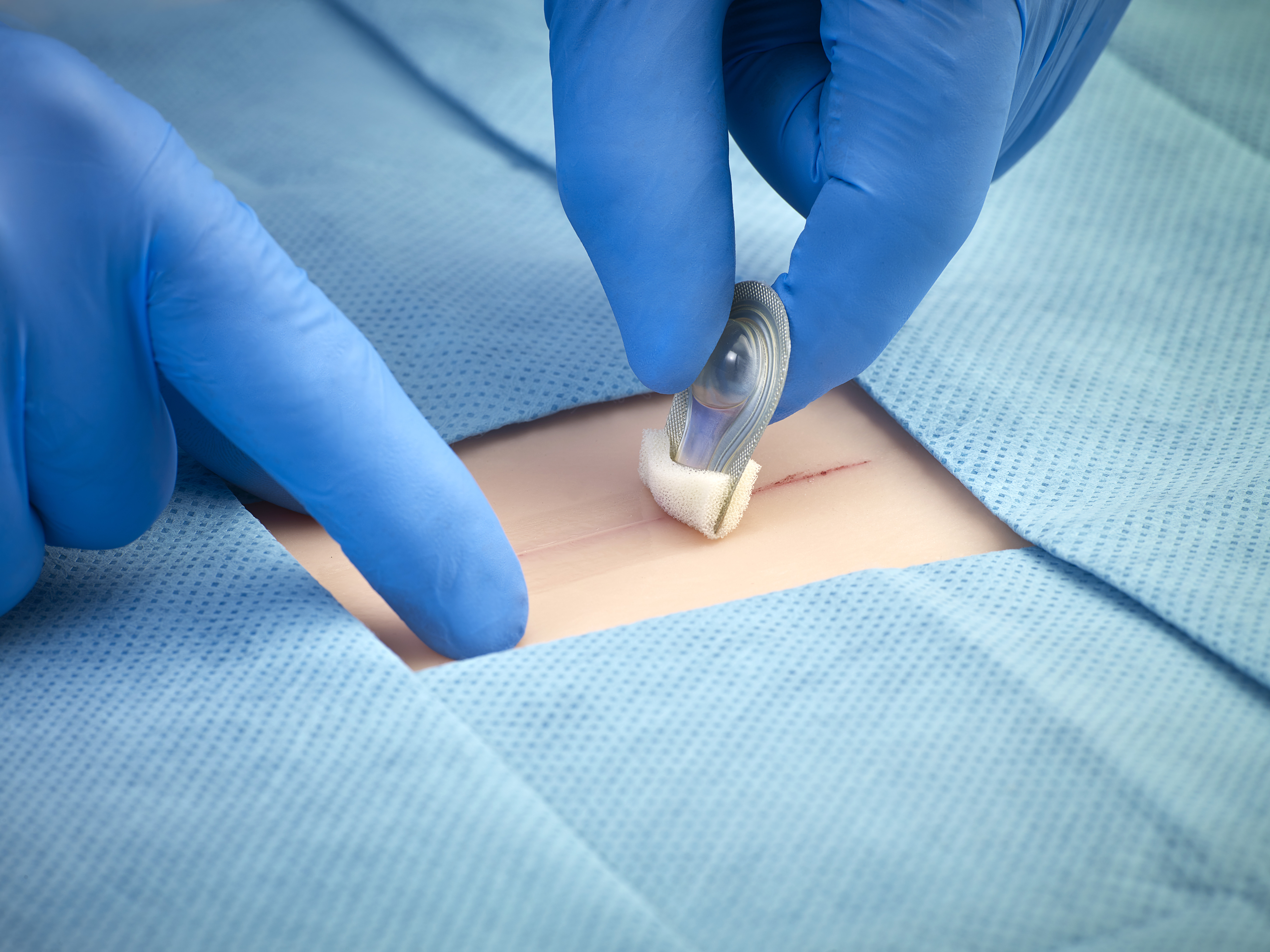
The proprietary formulation is designed to provide optimal permeability, increasing moisture and oxygen transmission to the wound. - Rapid Polymerization
Sets quickly—within minutes—saving time during wound closure procedures. - Microbial Barrier and Water-Resistant Protection
SurgiSeal adhesive forms a barrier on the skin, preventing bacteria and water from reaching the wound, reducing the risk of infection and allowing patients to shower immediately.2 - Low Exothermic Reaction
SurgiSeal adhesive is formulated with 2-octyl-cyanoacrylate, a longer alkyl chain that slows polymerization and minimizes heat release. This controlled reaction helps avoid sudden heat release. SurgiSeal’s low exothermic profile is a deliberate design choice to ensure safety, patient comfort and effectiveness in clinical settings. - Flexible Without Plasticizers
Maintains flexibility without added plasticizers, preserving adhesive integrity.
General surgery
Emergency Room
Operating Room
Urgent Care, Pediatrics
Featured Products
Explore our full range of both internal and external medical-grade solutions and discover how we are redefining performance in wound closure, vascular access, liquid embolics and infection control.
Our Associations


References
1 https://www.ajicjournal.org/article/S0196-6553(17)30861-1/abstract
2 Prince D, Slanki K, Varughese R, et al. Antibacterial effect and proposed mechanism of action of a topical surgical adhesive. Am J Infect Control. 2018;46:26–29. doi.org/10.1016/j.ajic.2017.07.008.
3 Schwade, Nathan D. eMedicine. 2007. WebMD. 17 September 2007, www.eMedicine.com